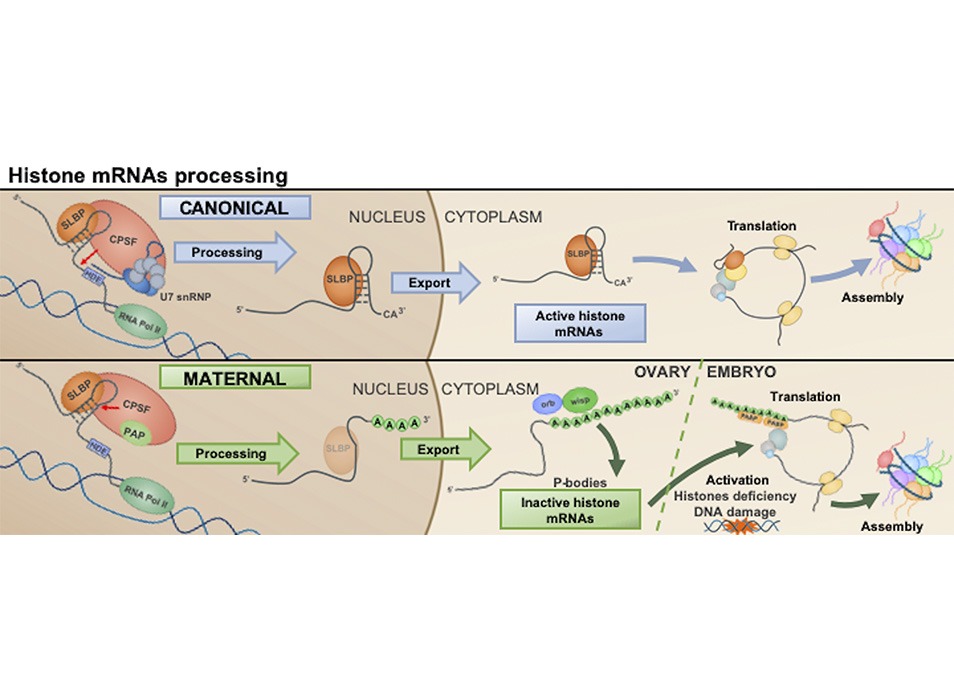
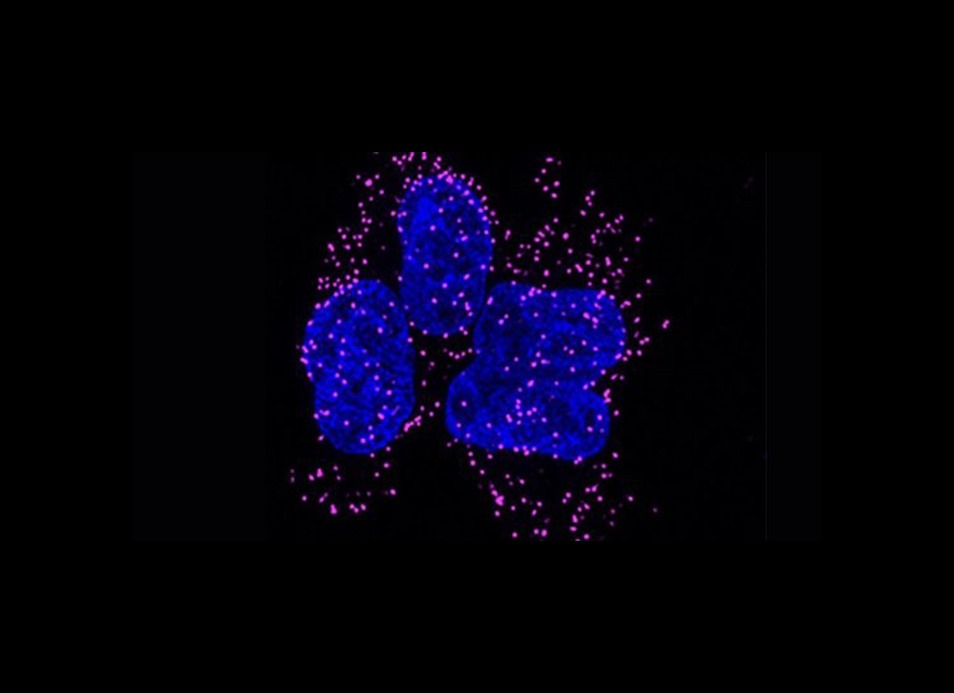
Histone transcripts are the only non-polyadenylated cellular mRNAs. They contain a highly conserved 3’UTR stem-loop…
Structures of pMV158 replication initiator RepB with and without DNA reveal a flexible dual-function protein
A new hexameric and DNA-bound structure of RepB, the replication initiation protein of plasmid pMV158, which confers antibiotic resistance, has been described in a paper published in Nucleic Acids Research. The structure reveals the great flexibility of the protein and how it recognizes the DNA at the origin of replication.
Abstract
Antibiotic resistance has become a global health threat. RepB is the rolling circle replication initiation protein of pMV158 plasmid, which contains a tetracycline resistance gene. The plasmid can be transferred to a wide range of bacteria, thus disseminating antibiotic resistance. A new hexameric structure of RepB has been described, which points at the great flexibility this protein displays. Flexibility is necessary for its dual role during plasmid replication, that is, to bind to two distinct positions of the plasmid and to cut one of the DNA strands to separate it, thereby initiating replication. A biochemical analysis and the crystal structure of the complex of RepB with dsDNA from the origin of replication shows how the protein recognizes the DNA.
This work is a collaboration between the labs of Prof. Miquel Coll at the Molecular Biology Institute of Barcelona (IBMB-CSIC) and the Institute for Research in Biomedicine (IRB Barcelona), and Prof. Gloria del Solar, at the Center for Biological Research (CIB-CSIC).
Reference:
Structures of pMV158 replication initiator RepB with and without DNA reveal a flexible dual-function protein. Machón, C., Ruiz-Masó, J.A., Amodio, J., Boer, D.R., Bordanaba-Ruiseco, L., Bury, K., Konieczny, I., del Solar, G. and Miquel Coll. Nucleic Acids Research (2023), DOI: https://doi.org/10.1093/nar/gkac1271