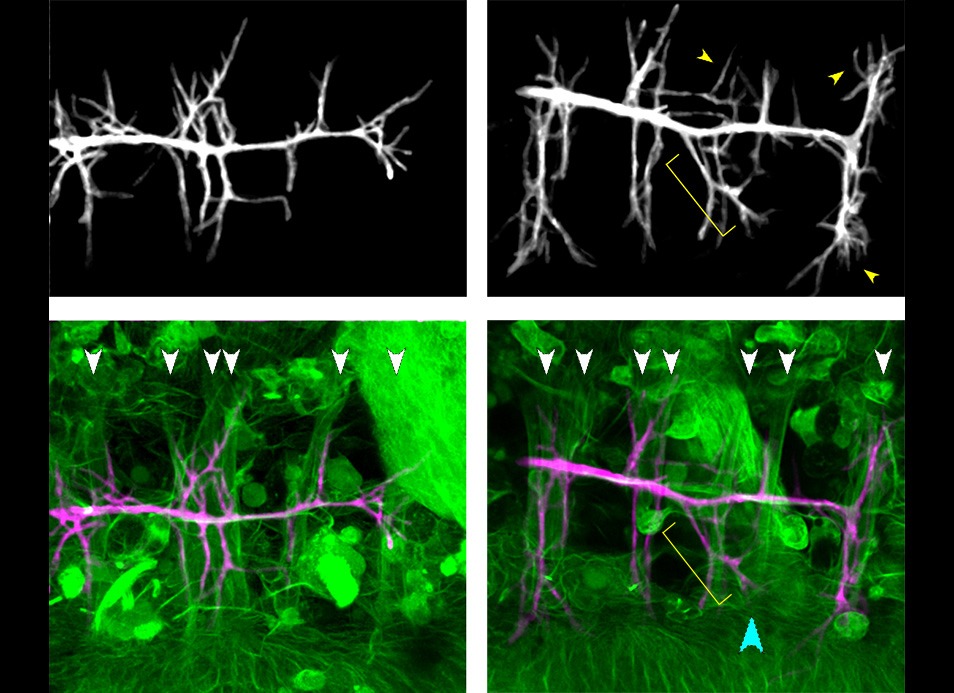
In embryos, epithelial sealing proceeds with progressive zipping eventually leading to a scar-free epithelium and…
‘Mitotic’ kinesin-5 is a dynamic brake for axonal growth in Drosophila
During neuronal development, microtubule reorganization shapes axons and dendrites, establishing the framework for efficient nervous system wiring. Our previous work has demonstrated the role of kinesin-1 in driving microtubule sliding, which powers early axon outgrowth and regeneration in Drosophila melanogaster. Here, we reveal a crucial new role for kinesin-5, a mitotic motor, in modulating postmitotic neuron development. The Drosophila kinesin-5, Klp61F, is expressed in larval brain neurons, with high levels in ventral nerve cord (VNC) neurons. Knockdown of Klp61F in neurons leads to severe adult locomotion defects and lethality, primarily due to defects in VNC motor neurons. Klp61F depletion results in excessive microtubule penetration into the axon growth cone, causing significant axon growth defects in culture and in vivo. These defects are rescued by a chimeric human-Drosophila kinesin-5 motor, indicating a conserved role for kinesin-5 in neuronal development. Altogether, we propose that kinesin-5 acts as a brake on kinesin-1-driven microtubule sliding, ensuring proper axon pathfinding in growing neurons.
Reference:
‘Mitotic’ kinesin-5 is a dynamic brake for axonal growth in Drosophila
Wen Lu, Brad S. Lee, Helen Xue Ying Deng, Margot Lakonishok, Enrique Martin-Blanco,Vladimir I. Gelfand
Development (2025) 152 (9): dev204424. https://doi.org/10.1242/dev.