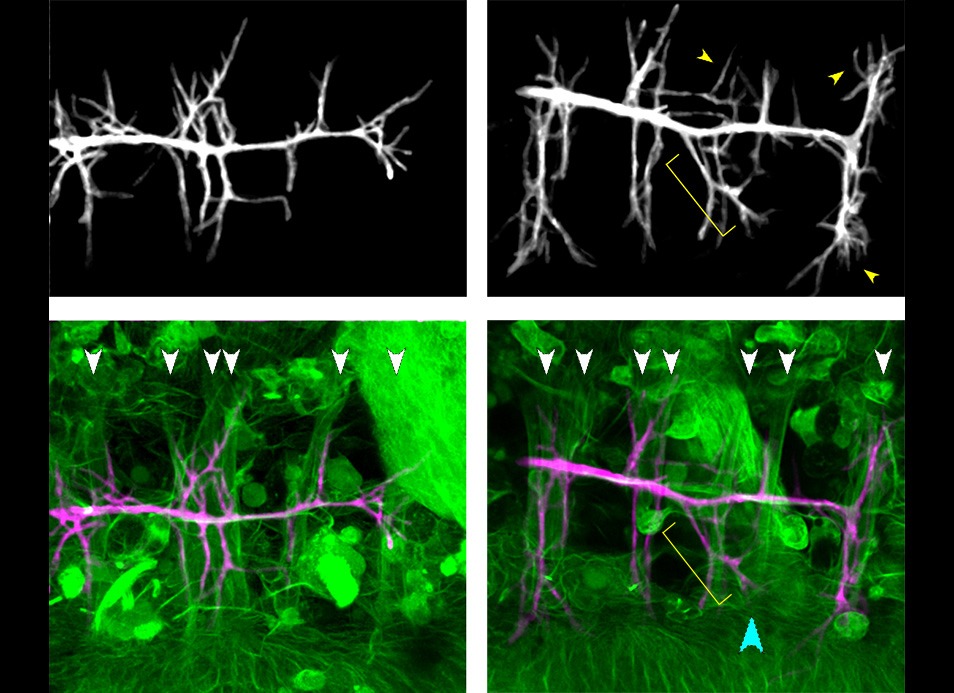
During neuronal development, microtubule reorganization shapes axons and dendrites, establishing the framework for efficient nervous…
Autocrine Wingless constricts the Drosophila embryonic gut by Ca+2- mediated repolarisation of mesoderm cells
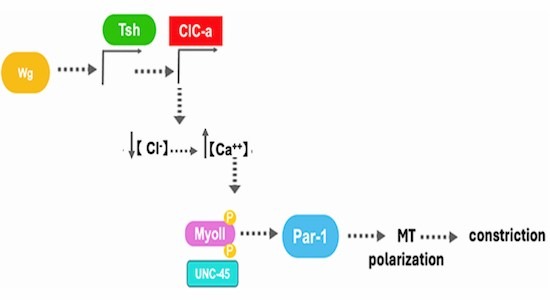
Besides the well-known role of Wg in in patterning, cell fate, or cell proliferation, this study describes the role of Wg in shaping organs or body parts. In particular, an autocrine Wg signal alters the polarity of the mesoderm via a Ca+2-mediated mechanism, which leads to the mesoderm constriction than then shapes the neighbouring endoderm.
Abstract
Wg/Wnt signalling —a highly conserved transduction pathway— has most commonly been found to be involved in patterning, cell fate, or cell proliferation, but less so in shaping organs or body parts. A remarkable case of the latter is the role of Wg signalling in the midgut of the Drosophila embryo. The Drosophila embryonic midgut is divided into four chambers that arise by the formation of three constrictions at distinct sites along the midgut. In particular, Wg is responsible for the middle constriction, a role first described more than 30 years ago. However, while some partial data have been obtained regarding the formation of this gut constriction, an overall picture of the process is lacking. Here we unveil that Wg signalling leads to this constriction by inducing ClC-a transcription in a subset of mesodermal cells. ClC-a, encodes a chloride channel, which in turn prompts a Ca+2 pulse in these cells. Consequently, the mesoderm cells, which already showed some polarity, repolarise and in so doing so they reshape the microtubule organisation, therefore inducing the constriction of the cells.
Reference:
Ricolo, D., Tamba, F. and Casanova, J. (2025) Autocrine Wingless constricts the Drosophila embryonic gut by Ca+2-mediated repolarization of mesoderm cells. EMBO Reports. https://doi.org/10.1038/s44319-025-00411-x